Problem statement
Which water sample has the highest BOD level?
Aim
To compare the level of biochemical oxygen demand (BOD) present in various water samples
Hypothesis
Drain water has the highest BOD level.
Variables
Manipulated variable: Water samples
Responding variable: BOD level
Constant variables: Volume of water and volume of methylene blue solution
Materials
Labelling paper, water samples from various sources (tap water, drain water, river water, pond water and well water), distilled water, 0.1% methylene blue solution
Apparatus
Reagent bottles with cap, 1 ml syringe with needle, stopwatch, marker pen, measuring cylinder
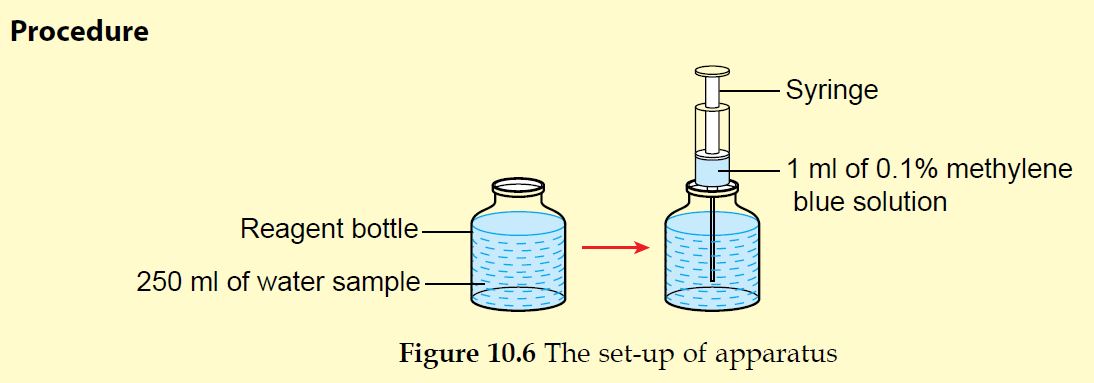
1. Get five water samples, each 250 ml, from five different sources (tap water, drain water, river water, pond water and well water).
2. Pour each water sample into separate reagent bottles until full. Label every reagent bottle (Figure 10.6).
3. Using a syringe, add 1 ml of 0.1% methylene blue solution into each water sample.
4. Put the cap of each reagent bottle immediately and place all the bottles in a dark cupboard. Make sure the reagent bottles are not shaken.
5. Repeat steps 2 to 4 using distilled water.
6. Check the reagent bottles every one hour for the next four hours until 0.1% methylene blue solution is decolourised.
7. Record the time for the 0.1% methylene blue solution to decolourise.
8. The rate of water pollution is calculated using the formula:

Results
Discussion
1. Explain the reason why the end of the syringe must reach the bottom part of the reagent bottle when putting in the 0.1% methylene blue solution.
2. From the experiment, which one of the water samples
(a) takes the fastest time for the 0.1% methylene blue solution to decolourise?
(b) is the slowest for the 0.1% methylene blue solution to decolourise?
3. Compare the BOD levels of all five water samples.
4. Why is this experiment being repeated using distilled water?
5. What is the relationship between time taken for 0.1% methylene blue solution to decolourise and the level of water pollution?
Conclusion
Is the hypothesis accepted? Suggest a suitable conclusion.
Answer:
Discussions:
1. The end of the syringe must reach the bottom part of reagent bottle to avoid the solution from being oxidised by atmospheric oxygen in the reagent bottle.
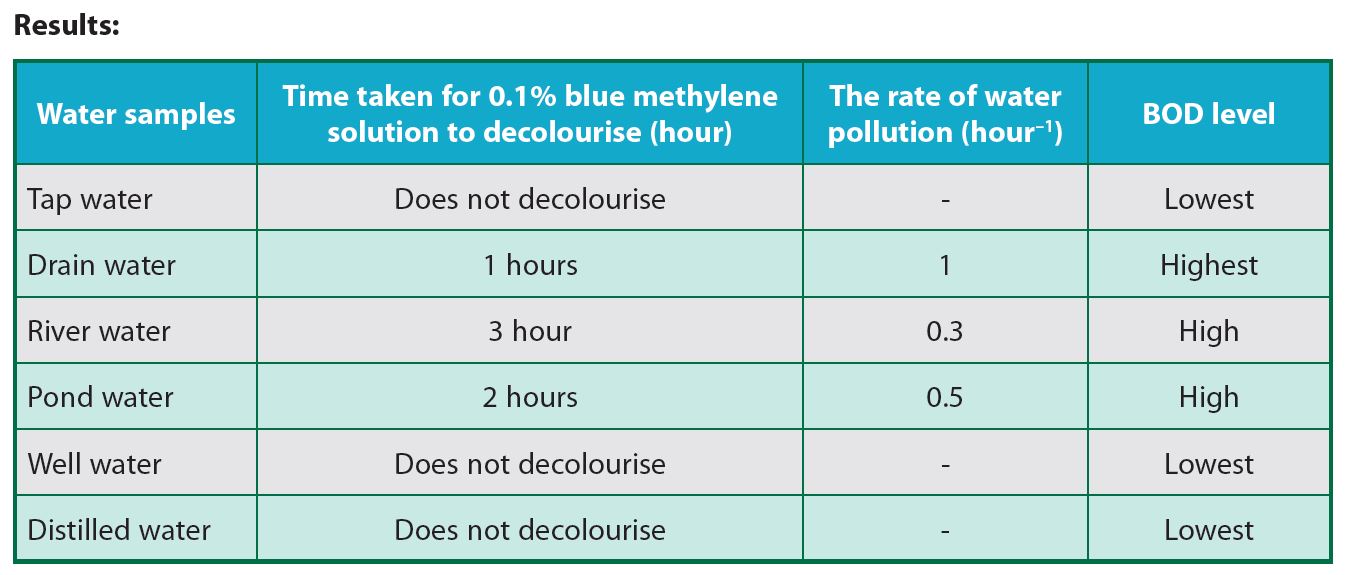
2.(a) The fastest for the 0.1% methylene blue solution to decolourise: Drain water
2.(b) The slowest for the 0.1% methylene blue solution to decolourise: Tap water
3. Drain water shows the highest BOD level followed by pond water, river water, well water and tap water. Drain water takes the fastest time to decolourise 0.1% methylene blue solution as compared to the other samples. Drain water contains the lowest dissolved oxygen content as compared to the other samples. This showed that the drain water is the most polluted.
4. As a control set
5. The faster the time taken to decolourise the 0.1% methylene blue solution, the higher the level of water pollution.